Quercefit® – Quercetin Indena Phytosome™
Browse all Indena’s documents about products, events, company information and so much more.
Go to sectionQuercefit® provides an optimized quercetin solubility, proving up to 20-fold more bioabsorbed than unformulated quercetin, remaining in line with a diet rich in vegetables and fruits. Therefore, it can be used at lower dosages, while still offering the benefits of a quercetin full serving.
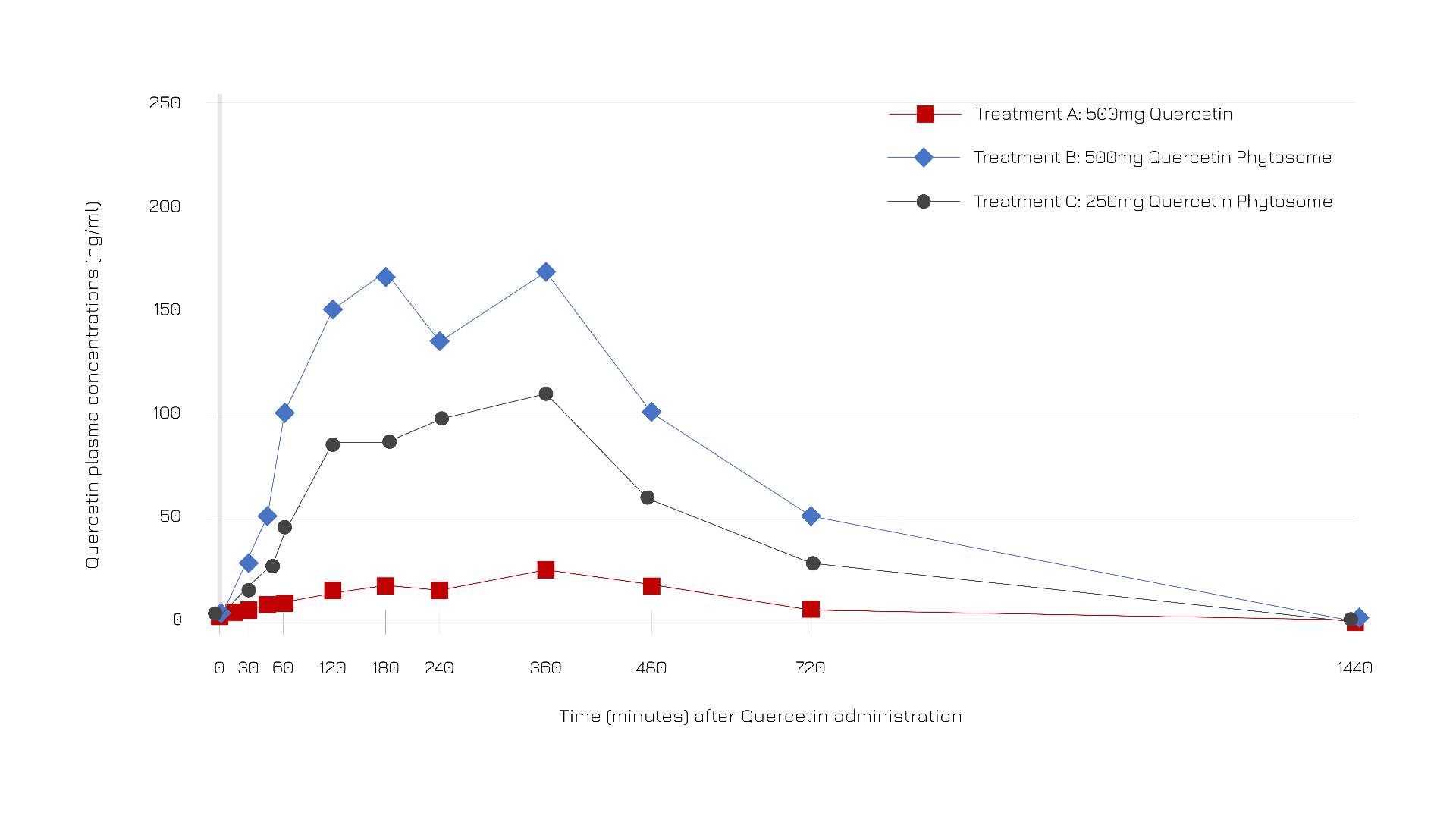
According to a trial on 12 participants (aged 18-50) supplemented with one oral dose of either 500 mg unformulated quercetin, 250 mg of Quercefit® or 500 mg of Quercefit®, Quercefit® optimizes quercetin plasma levels (Figure 1).
Figure 1: Time-evolution of quercetin plasma concentrations (ng/ml) after oral administration of unformulated quercetin (red), Quercefit® 250 mg (gray) and Quercefit® 500 mg (blue).
Peer-reviewed science on Quercefit®
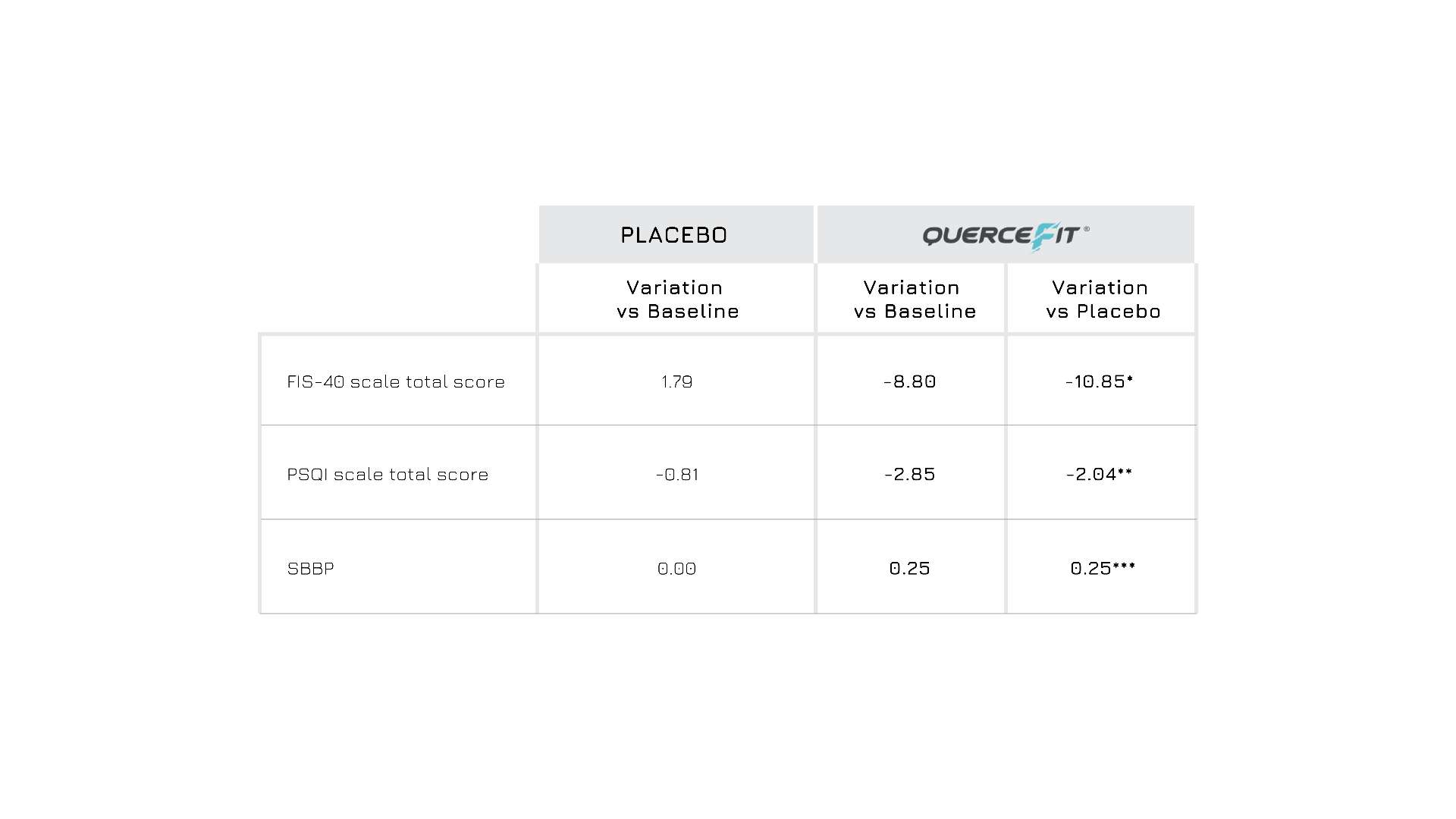
A double-blind, randomized, placebo-controlled human study recruited 78 healthy individuals with chronic fatigue-like symptoms to evaluate the positive effects of supplementing Quercefit® (500 mg/day) after a 2-month period from the baseline visit. Data from the Quercefit® group showed significant improvements in all parameters measured, including fatigue score (FIS-40 scale), sleep quality (PSQI scale) and physical performance (SPPB test), compared to both the baseline and the placebo group (Figure 2).
In a trial conducted on 48 non-professional triathlon athletes, Quercefit® showed positive effects on training, performance and ache relief: the group supplemented with Quercefit® ran faster (-11.3% time from start to finish line, compared to -3.9% in the control group). In addition, post-run muscular discomfort, cramps and recovery time all improved with Quercefit®, and oxidative stress was balanced.
Figure 2: Variation of FIS-40, PSQI and SBBP in placebo group and Quercefit® group vs baseline, after 2 months of supplementation. *p<0.0001 vs placebo; ** p<0.001 vs placebo; ***p<0.001 vs placebo.
Quercefit® may exert functions as a senolytic agent by supporting healthy cell turnover: on top of its strong antioxidant activity, quercetin may play a key role in longevity, contributing to a positive healthspan.
By promoting cellular renewal and a healthy inflammatory response, it nurtures beauty from within—helping to reveal a healthier, more radiant you on the outside.
Quercefit® shows a potential promising effect during allergy season. A further study confirms that after 30 days, administration optimizes general wellbeing in allergy-prone subjects and modulates rhinitis, in addition to reducing the frequency of intermittent discomforts up to 50% during the day and up to 70% during the night.
In another study on Japanese subjects, repeated oral intake of a Quercefit®-containing supplement for 4 weeks has been shown to reduce allergy-related symptoms (both for seasonal and annual allergens) such as eye itching, sneezing, nasal discharge and sleep disorders compared to the placebo group (n=66).
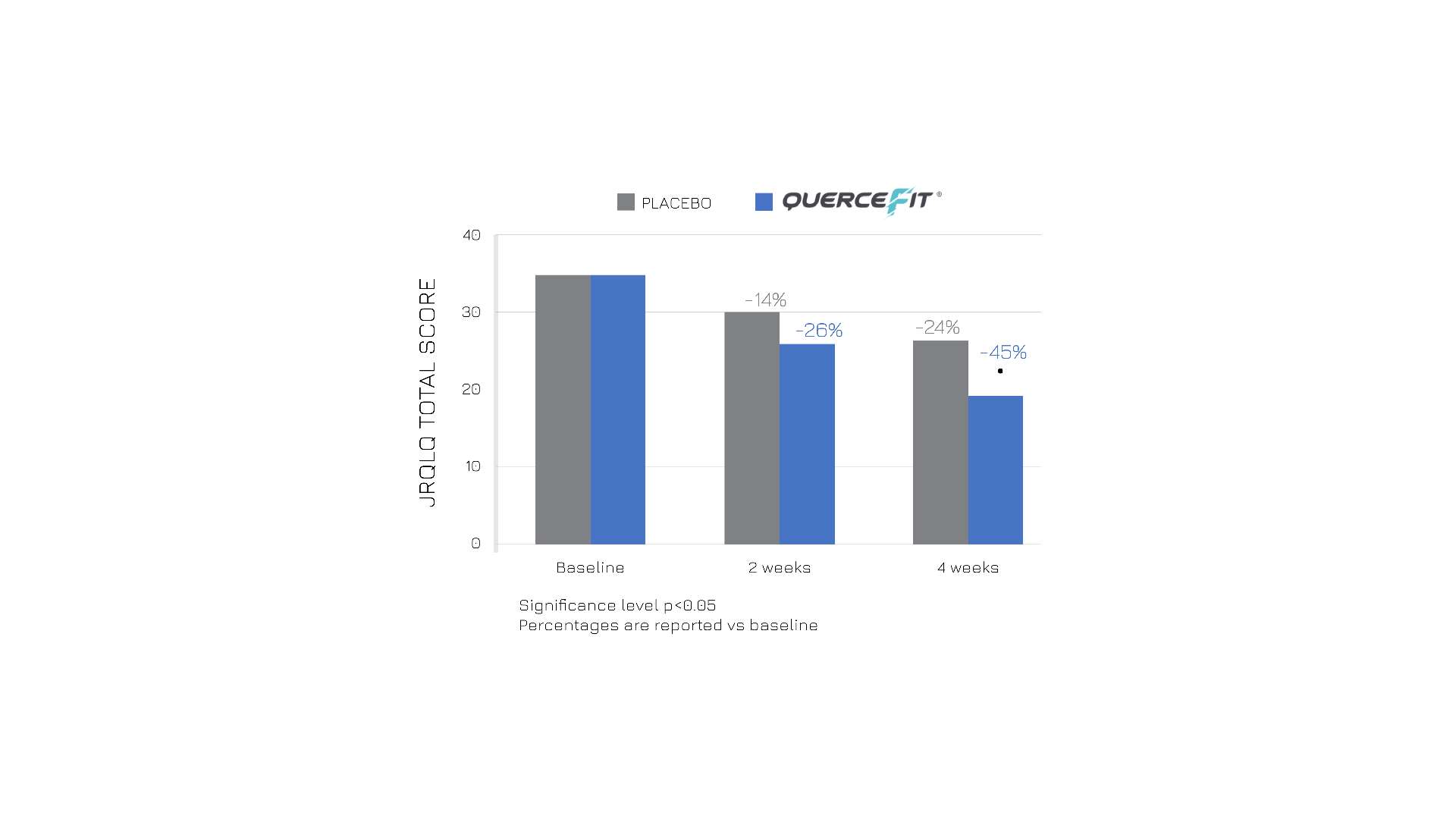
Moreover, the treated group also experienced improved quality of life, as reported in the chart below (Figure 3).
Early supplementation with Quercefit® is proven to positively modulate the immune system’s response upon exposure to allergens - in a validated, standard histamine test - resulting effective also in healthy subjects.
Figure 3: Total score of the Japanese Rhino-conjunctivitis Quality of Life Questionnaire in the two groups, before starting supplementation (baseline), and 2 and 4 weeks later. Graphic representation of ref.1 table III.
Exploiting the pleiotropic properties of quercetin thanks to Indena Phytosome™ technology, Quercefit® allows to counteract main symptoms emerging after virus exposure.
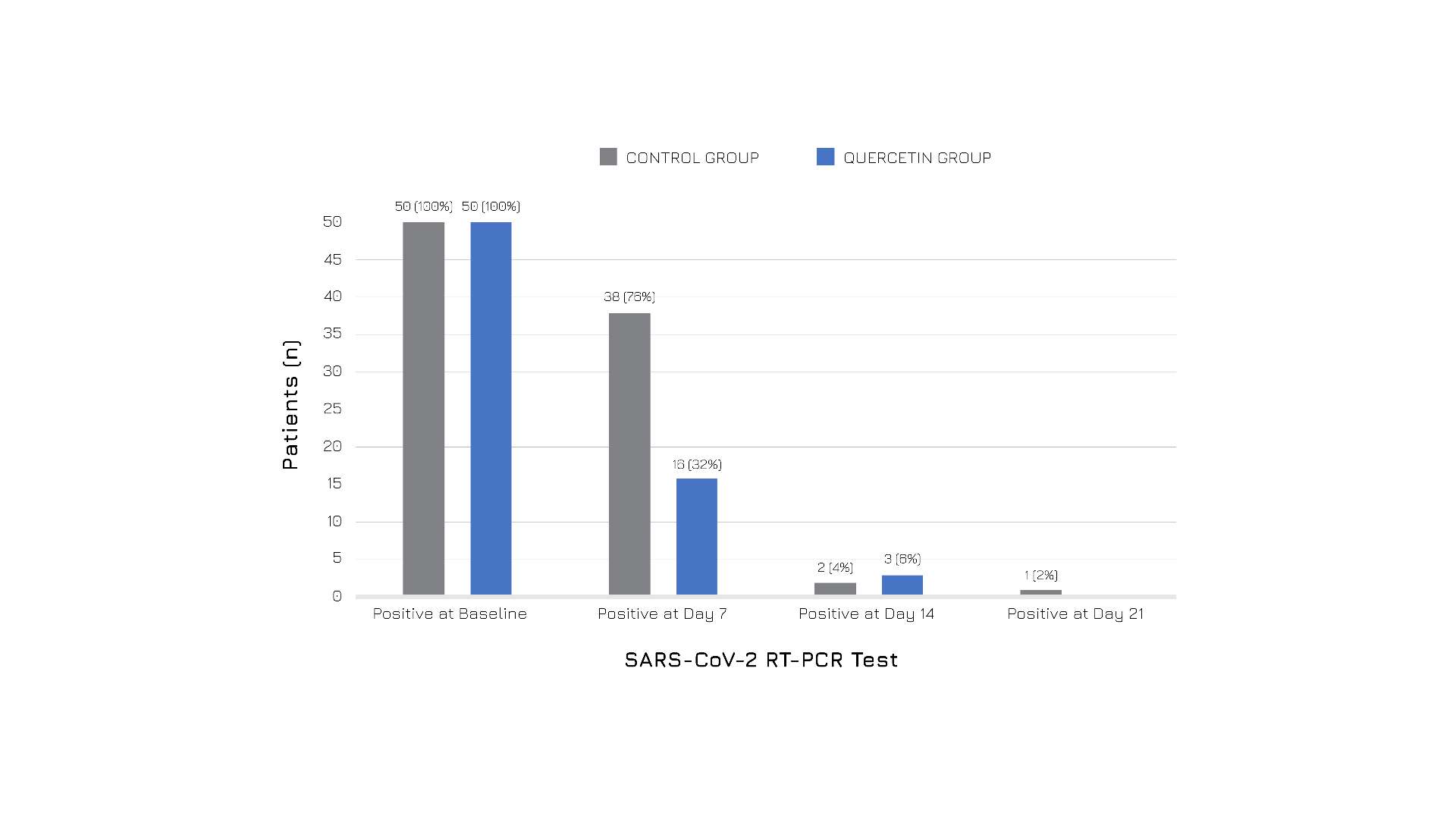
Quercefit® effectiveness has been valideted in multiple human studies in subjects tested positive for SARS-CoV-2.
Participants received either standard care alone or in combination with Quercefit® as an add-on supplement (500 mg 2 or 3 times daily).
The results showed that those who received Quercefit® alongside standard care experienced faster recovery and overall better health outcomes compared to the control group, in particular, the supplementation led to:
Figure 4: Subjects’ follow-up RT-PCR COVID-19 test results in the two groups.
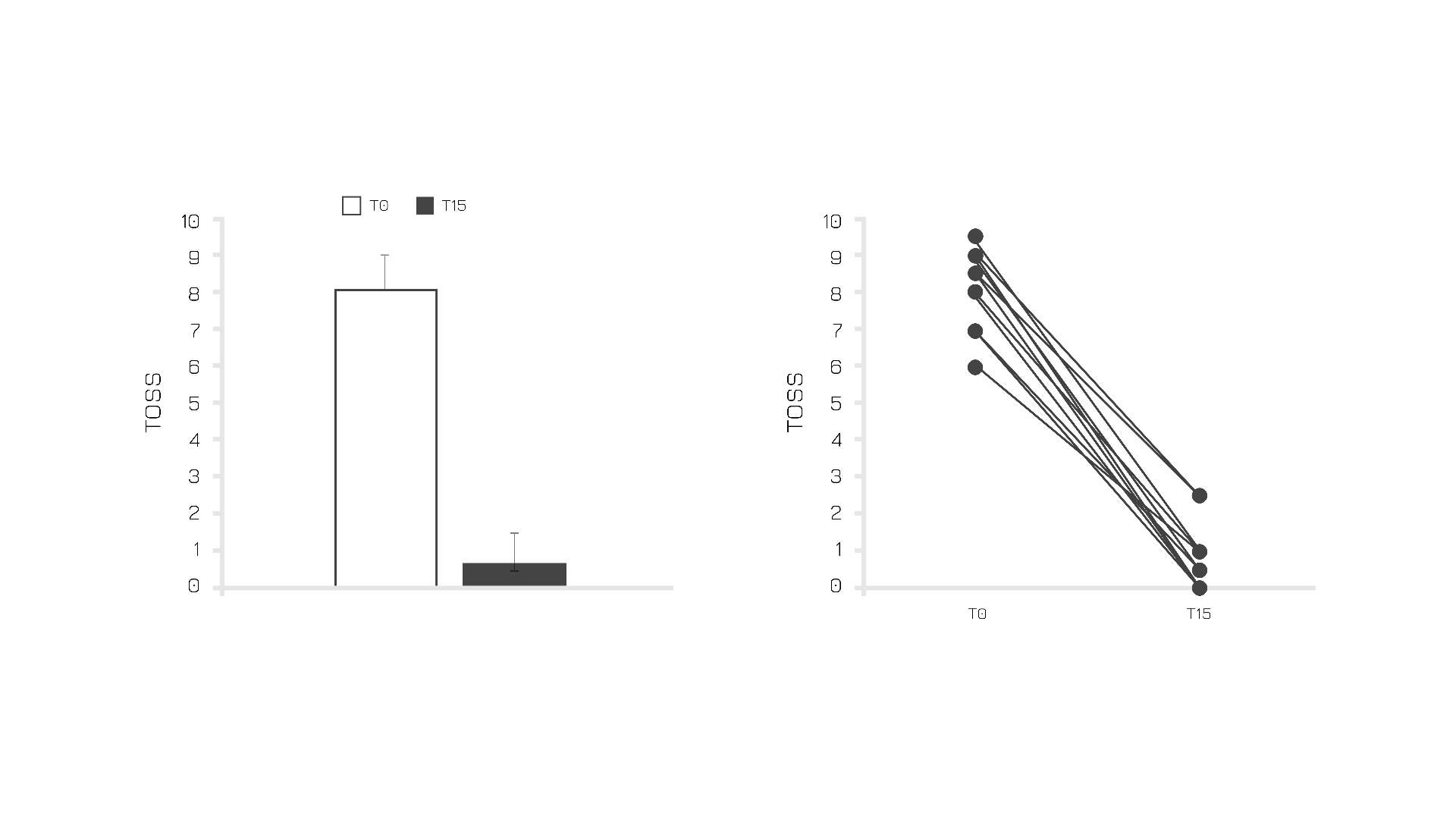
Confirming its beneficial role for ocular allergy-related disorders, a study performed on adults affected by allergic conjunctivitis showed that Quercefit® may potentially be the perfect natural aid to avoid clinical worsening. In particular, an analysis on 15 subjects after 15 days of supplementation (250 mg, twice a day) highlighted significant improvements in terms of Total Ocular Symptoms Score (TOSS) (Figure 5).
Figure 5: TOSS score before and after 15 days of Quercefit® supplementation.
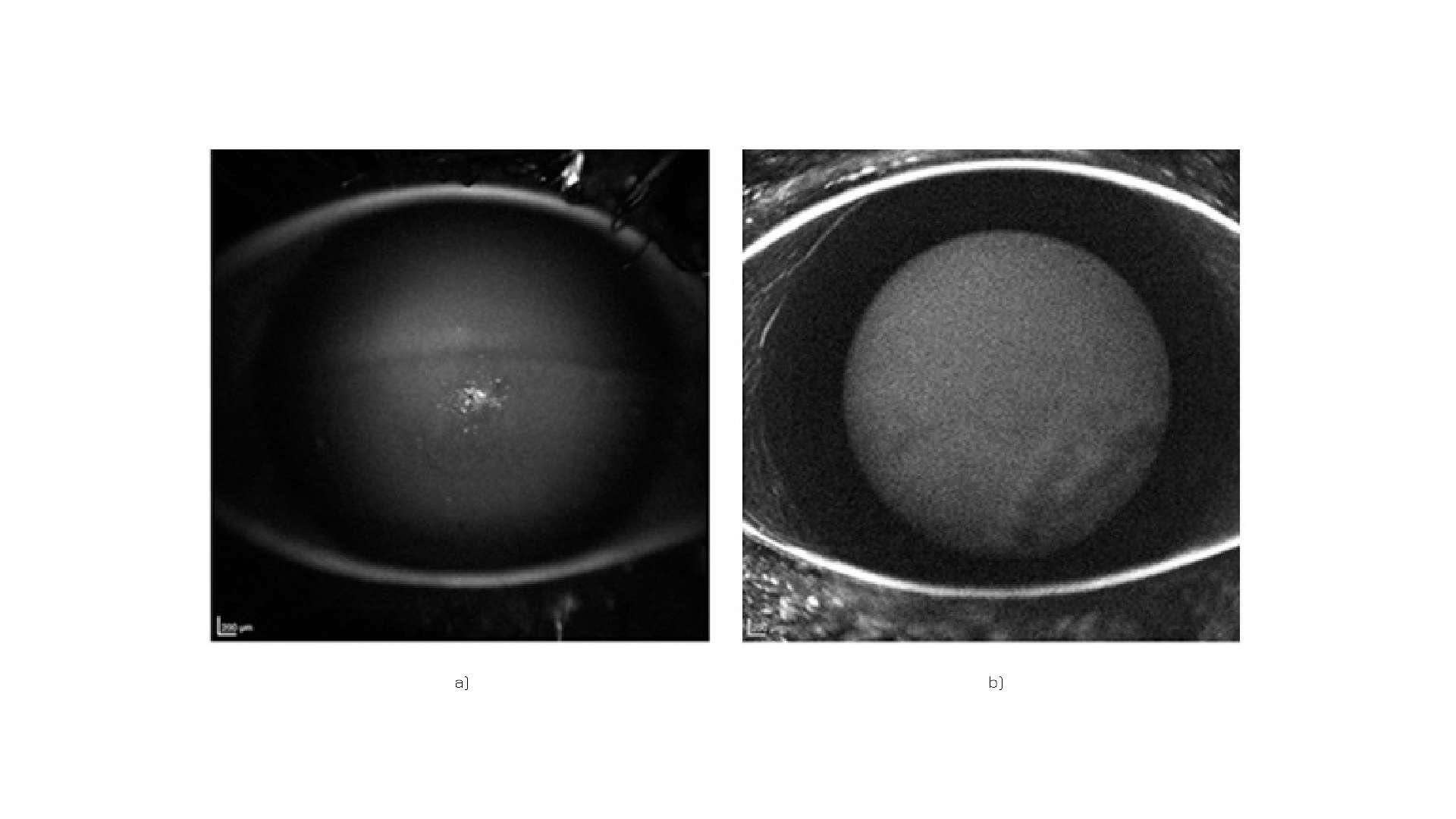
A series of 8 case studies have confirmed that Quercefit® can be the perfect ally for eye health, during topical antibiotic therapy on damaged ocular tissues (Figure 6).
Figure 6: Corneal condition following misuse of soft daily contact lenses, before (a) and after (b) supplementation.
BIBLIOGRAPHY
1 Riva A. et al.; Eur J Drug Metab Pharmacokinet (2019): 44(2): 169-77.
2 Rondanelli M.; et al.; Biomedicine & Pharmacotherapy 167 (2023): 115453.
3 Riva A. et al.; Minerva Medica (2018); 109(4): 285-9.
4 Lee S., et al., Nature (2025): 638 (8052): E46.
5 Cesarone, M. R., et al.; Minerva medica (2019): 110(6): 524-9.
6 Belcaro G., et al., Esperienze Dermatol (2020): 22(1): 5-9.
7 Yamada, S. et al., Eur Rev Med Pharmacol Sci (2022): 26(12): 4331-45.
8 Di Pierro F. et al.; Int J Gen Med 14 (2021): 2807-16.
9 Di Pierro F. et al; Frontiers in Pharmacology (2023): 13(13): 1096853.
10 Rondanelli M. et al.; Life (2022): 12(1): 66.
11 Mazzolani et al.; Austin J allergy (2022): 8(1): 1042.
12 Riva A. et al.; Int J Clinical & Case (2022): 6(3): 10-9.
Sorry, our website doesn't support IE11 and older versions
For a better experience try a modern browser:
This is a private file, to request the download of this resource, please fullfill the fields below.