Berbevis® – Berberine Indena Phytosome™
Browse all Indena’s documents about products, events, company information and so much more.
Go to sectionA pharmacokinetic study has shown Berbevis® greatly favors berberine’s bioaccessibility profile (AUC) on molar basis and with observed dose linearity. The pharmacokinetic profile of total and free berberine after the administration of Berbevis® and of berberine chloride to healthy volunteers was tested in a study with twelve healthy volunteers of both sexes with a mean age of 29 ± 7.72 and a mean BMI of 23.09 ± 1.27.
A human randomized-controlled, double-blind, versus placebo trial with Berbevis® showed that a 60-day supplementation may help support the balance of blood sugar levels in individuals with challenged fasting glucose. Participants who took the ingredient experienced a favorable shift in fasting glycemia compared to those receiving the placebo.
Lipid values also followed a positive trend. Total cholesterol, triglycerides, and the cholesterol/HDL ratio moved in a more balanced direction in the supplemented group, suggesting a potential benefit for metabolic well-being.
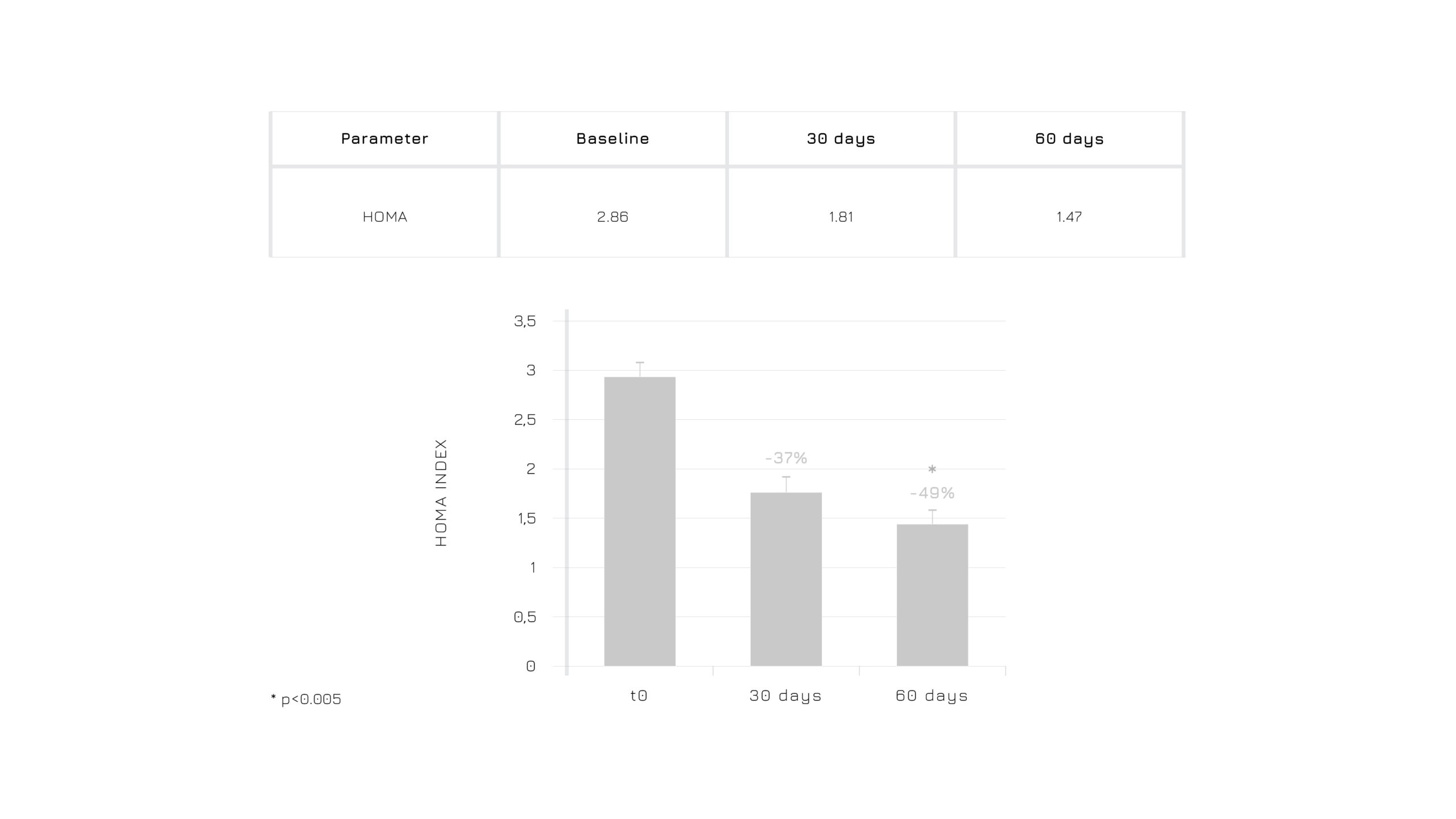
Regarding insulin and glucose management, the data indicated a more favorable pattern in both insulin levels and the HOMA index—an important marker of insulin resistance.
Furthermore, the ApoB/ApoA ratio and total cholesterol values, showed a meaningful adjustment in the Berbevis® group: this shift may reflect a healthier lipid profile, which could contribute to maintain an overall lipid metabolism in shape.
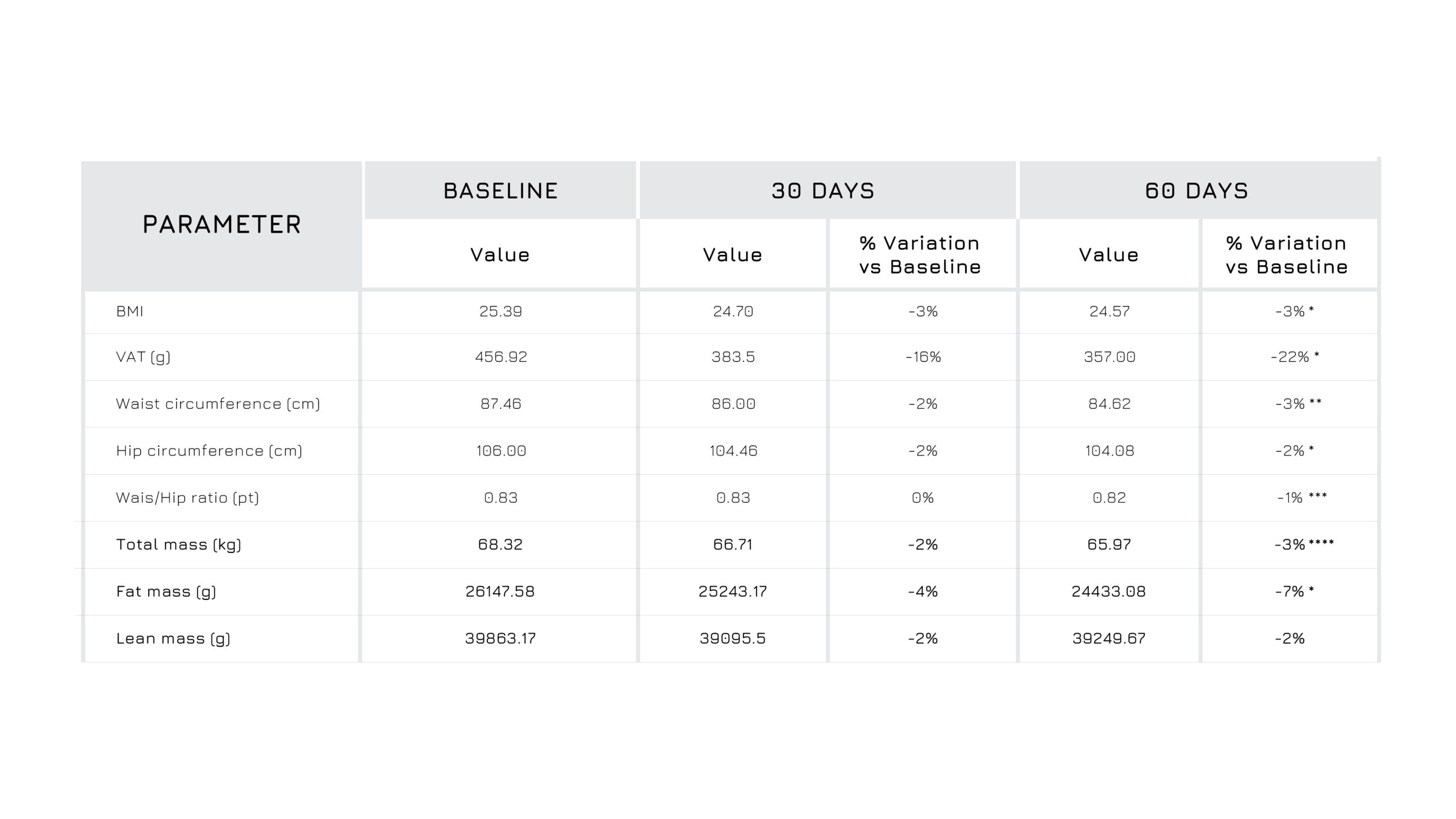
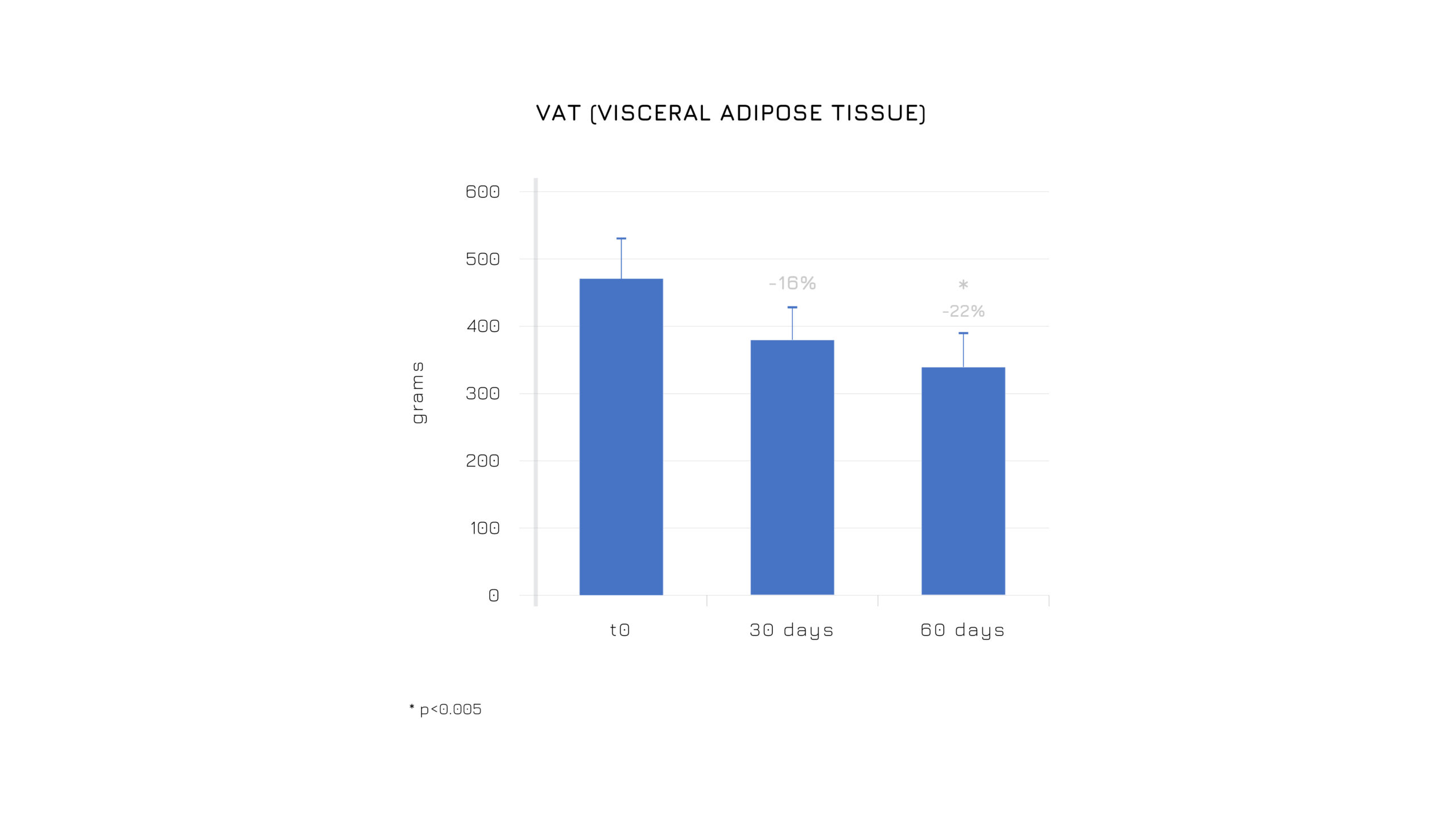
In terms of body composition, participants receiving Berbevis® showed a tendency toward lower levels of: visceral fat, total fat mass and waist circumference.
The supplementation was well tolerated throughout the study, with no reported side effects.
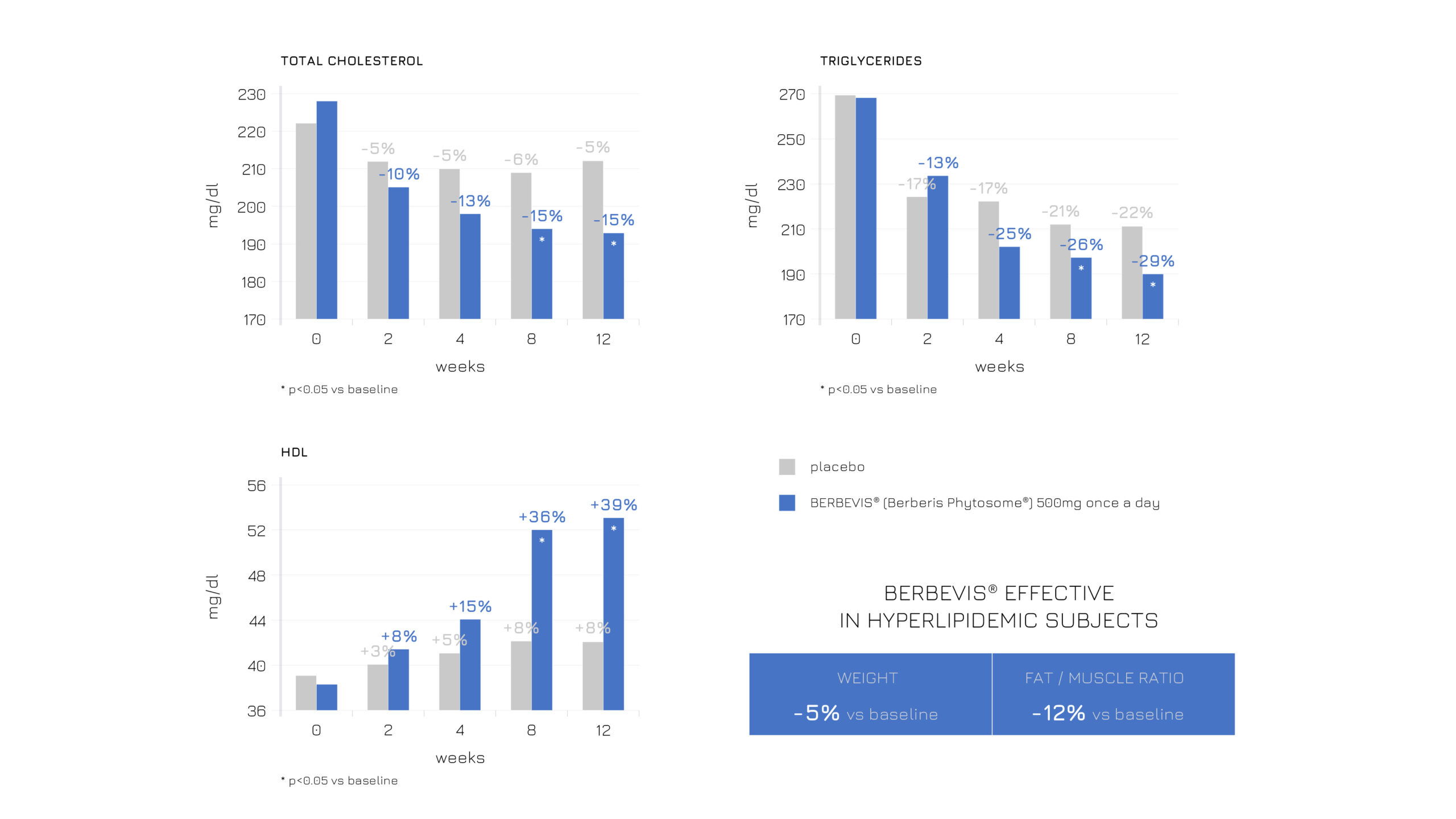
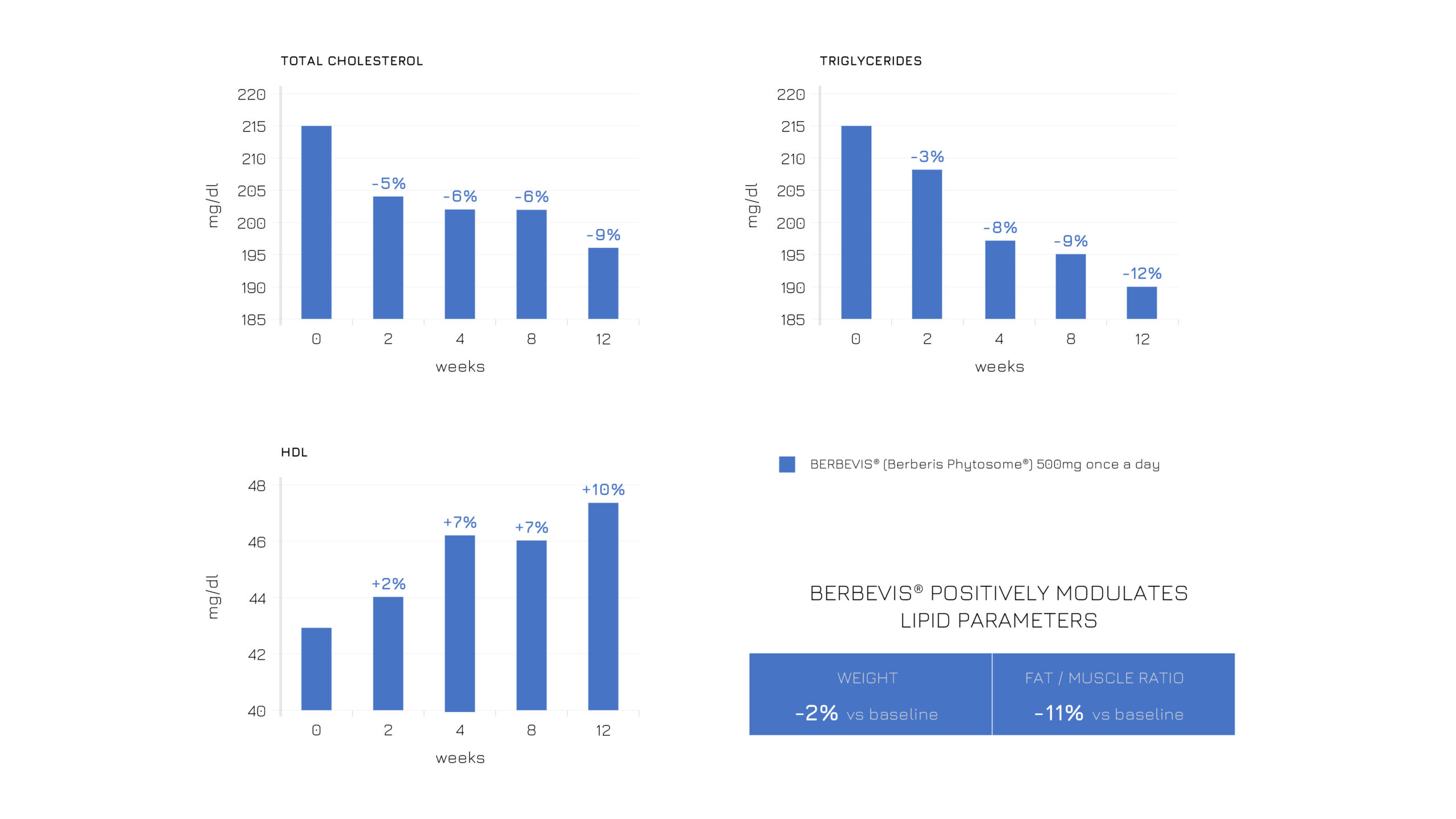
Berbevis® effectiveness in balancing the blood lipid profile and modulating body composition was proven also in borderline hyperlipidemic subjects supplemented with a single daily 550-mg dose.
Specifically, two different populations were enrolled in the study: subjects with stable hyperlipidemia (n=47; Standard Management + Berbevis® vs Standard Management) and with occasional hyperlipidemia (n=25; Standard Management + Berbevis® vs baseline).
In both cases, Berbevis® rebalanced blood lipids, including T-Col, HDL and triglycerides, with a positive outcome on anthropometric measurements (see Figures 1 and 2).
A recent clinical study also validated Berbevis® as a precious ally for cardiovascular health, being able to rebalance major parameters related to atherosclerotic risk (e.g. endothelial function and homocysteine) in hyperlipidemic subjects supplemented with 550 mg/day for 6 months.
This result confirms Berbevis® efficacy and safety even for long-term supplementation and endorses it as a valuable shield protecting cardio-metabolic health.
Figure 1: Effects of a single daily dose of Berbevis® in stably hyperlipidemic subjects, considering HDL, total cholesterol, TG and body composition (weight and fat/muscle ratio).
Figure 2: Effects of a single daily dose of Berbevis® in occasionally hyperlipidemic subjects, considering HDL, total cholesterol, TG and body composition (weight and fat/muscle ratio).
Figure 3. Descriptive statistics for the body composition measured at baseline (t0) and after 60 days (t2) of Berbevis® supplementation.W/H: Waist/Hip Ratio; BMI: Body Mass Index. *p<0.05; **p<0.005.
Figure 4. Effectiveness on body composition, inducing a redistribution of adipose tissue with the reduction of the visceral fat tissue and fat mass. *p<0.005.
Figure 5. Effect of Berbevis® on HOMA index in PCOS individuals (*p≤0.005).
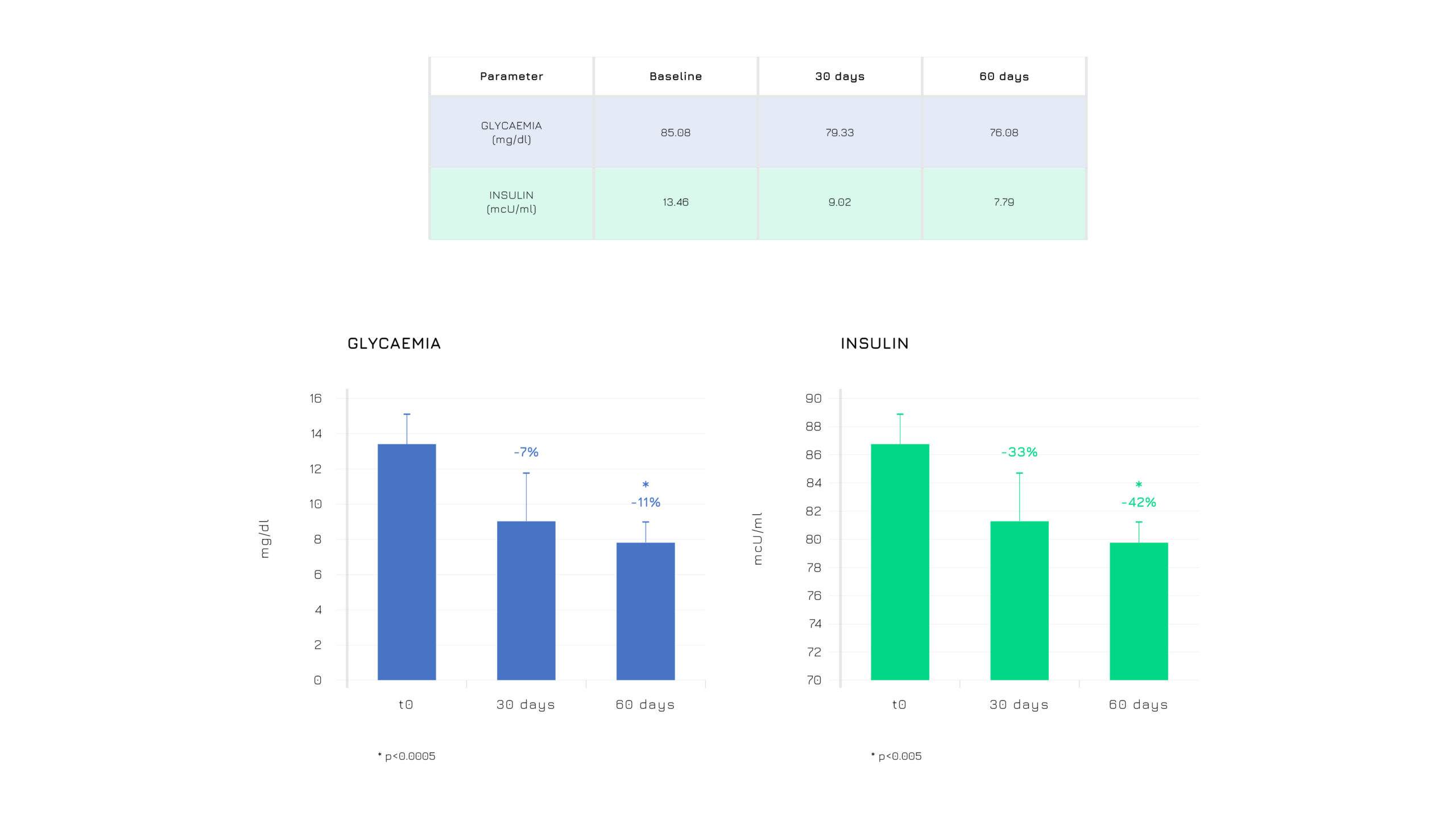
Berbevis® significantly improves blood sugar profile optimizing both glucose and insulin (Figure 6)2.
Figure 6. Effect of Berbevis® on the glycemic profile in PCOS individuals (*p≤0.005; **p<0.0005).
BIBLIOGRAPHY
1Petrangolini G. et al.; Evid Based Complement Alternat Med. Nov (2021)
2Rondanelli M. et al.; Eur Rev Med Pharmacol Sci. Jul;27(14):6718-6727 (2023).
3Cesarone MR. et al.; Minerva Gastroenterol (Torino).70(1):10-15. (2024).
4Cesarone, Maria R., et al. Minerva medica (2025).
5Rondanelli M. et al.; Nutrients. Oct 19;13(10):3665 (2021).
Sorry, our website doesn't support IE11 and older versions
For a better experience try a modern browser:
This is a private file, to request the download of this resource, please fullfill the fields below.